
The only FDA-cleared device to deviate the esophagus and reduce the risk of thermal injury during ablation for atrial fibrillation
esolution®
esolution is the first and only FDA-cleared device indicated for use in any ablation energy source to deviate the esophagus and reduce the risk of ablation-related esophageal injury during treatment of atrial fibrillation.
How it works
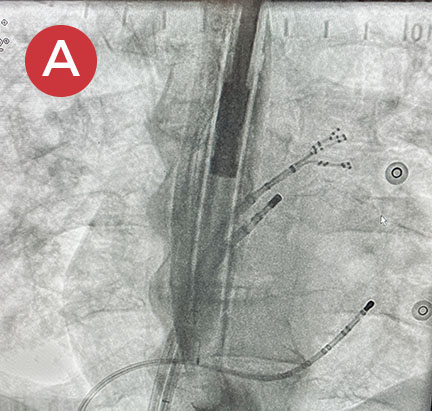
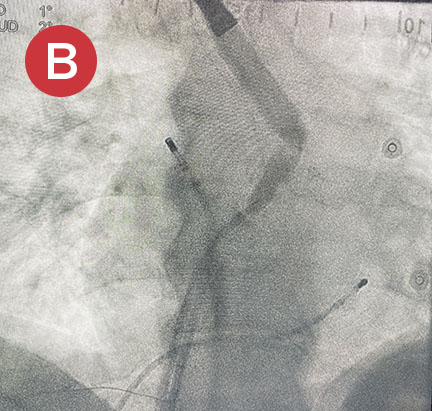
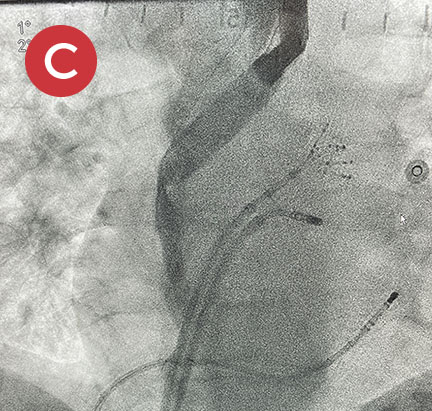
Ablate with Confidence™
The esolution device works by providing bilateral deviation to accommodate each patient’s pulmonary vein anatomy and any lesion strategy. This flexibility allows the electrophysiologist to determine when and where deviation is needed so that ablation can be performed with confidence anywhere in the left atrium.
Protecting the Esophagus
The esophagus lies close to the point of ablation energy treatment. Esophageal injury related to ablation has been reported in as high as 47% of patients undergoing AF ablation. Numerous approaches and devices have attempted to protect the esophagus, but only esolution is FDA-cleared to reduce esophageal injury using any ablation energy source.
Compatibility
esolution is the only device designed by electrophysiologists and developed specifically for catheter ablation workflow. esolution can be used with any ablation modality, and does not require any unique hardware, software, or other accessories that can slow down procedures. esolution is compatible with 9Fr temperature probes and will not interfere with mapping systems.